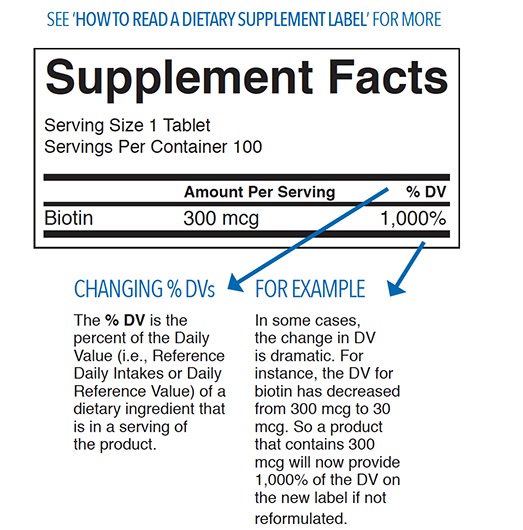
Proper labeling is not just about marketing—it’s a core FDA requirement. Labels must be truthful, not misleading, and must provide required information as per 21 CFR 101 and DSHEA.

Identity testing of all incoming dietary ingredients is strictly required and documented. Establish clear, written specifications for each component used.

Prevent mislabeling and ensure proper label reconciliation procedures. Finished product labels must consistently meet established specifications.
Get Our Latest Update & New Offers Sales Discount
Copyright © 2026, All Right Reserved.